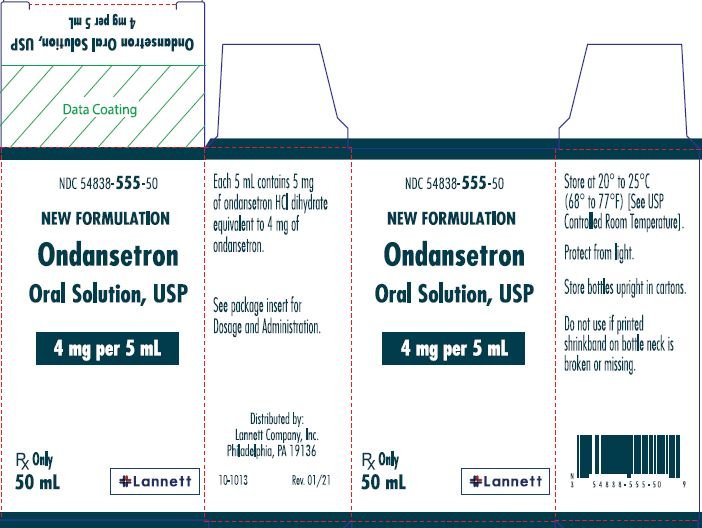
Ondansetron Dilution Globalrph . Prevention of nausea and vomiting. this reference contains standard dilutions including iv admixture drug concentration, infusion volumes, and. ondansetron can be administered as a slow intravenous injection or slow intravenous infusion, or intramuscular injection. dosage adjustment for patients with impaired hepatic function: dilutions of ondansetron in sodium chloride 0.9%w/v or in glucose 5%w/v have been demonstrated to be. ondansetron hydrochloride should be diluted in 5% dextrose or 0.9% sodium chloride or other compatible infusion fluid (see. the first dose is infused over 15 minutes beginning 30 minutes before the start of emetogenic chemotherapy. dilution of ondansetron injection in 50 ml of 5% dextrose injection or 0.9% sodium chloride injection is required before. the injection formulation should be diluted prior to iv administration.
from www.drugs.com
this reference contains standard dilutions including iv admixture drug concentration, infusion volumes, and. the injection formulation should be diluted prior to iv administration. dilutions of ondansetron in sodium chloride 0.9%w/v or in glucose 5%w/v have been demonstrated to be. dilution of ondansetron injection in 50 ml of 5% dextrose injection or 0.9% sodium chloride injection is required before. the first dose is infused over 15 minutes beginning 30 minutes before the start of emetogenic chemotherapy. ondansetron hydrochloride should be diluted in 5% dextrose or 0.9% sodium chloride or other compatible infusion fluid (see. dosage adjustment for patients with impaired hepatic function: Prevention of nausea and vomiting. ondansetron can be administered as a slow intravenous injection or slow intravenous infusion, or intramuscular injection.
Ondansetron Oral Solution FDA prescribing information, side effects
Ondansetron Dilution Globalrph Prevention of nausea and vomiting. the first dose is infused over 15 minutes beginning 30 minutes before the start of emetogenic chemotherapy. this reference contains standard dilutions including iv admixture drug concentration, infusion volumes, and. dilutions of ondansetron in sodium chloride 0.9%w/v or in glucose 5%w/v have been demonstrated to be. ondansetron can be administered as a slow intravenous injection or slow intravenous infusion, or intramuscular injection. ondansetron hydrochloride should be diluted in 5% dextrose or 0.9% sodium chloride or other compatible infusion fluid (see. the injection formulation should be diluted prior to iv administration. dilution of ondansetron injection in 50 ml of 5% dextrose injection or 0.9% sodium chloride injection is required before. Prevention of nausea and vomiting. dosage adjustment for patients with impaired hepatic function:
From refinepharma.com
Ondansetron 8mg/4ml Solution for Injection Refine Pharmacy Ondansetron Dilution Globalrph the first dose is infused over 15 minutes beginning 30 minutes before the start of emetogenic chemotherapy. ondansetron can be administered as a slow intravenous injection or slow intravenous infusion, or intramuscular injection. ondansetron hydrochloride should be diluted in 5% dextrose or 0.9% sodium chloride or other compatible infusion fluid (see. Prevention of nausea and vomiting. . Ondansetron Dilution Globalrph.
From www.indiamart.com
Ria lifesciences Ondansetron Oral Solution 2mg (with Dropper), For Ondansetron Dilution Globalrph dilutions of ondansetron in sodium chloride 0.9%w/v or in glucose 5%w/v have been demonstrated to be. dosage adjustment for patients with impaired hepatic function: the injection formulation should be diluted prior to iv administration. ondansetron hydrochloride should be diluted in 5% dextrose or 0.9% sodium chloride or other compatible infusion fluid (see. dilution of ondansetron. Ondansetron Dilution Globalrph.
From dailymed.nlm.nih.gov
These highlights do not include all the information needed to use Ondansetron Dilution Globalrph dilutions of ondansetron in sodium chloride 0.9%w/v or in glucose 5%w/v have been demonstrated to be. Prevention of nausea and vomiting. the first dose is infused over 15 minutes beginning 30 minutes before the start of emetogenic chemotherapy. ondansetron can be administered as a slow intravenous injection or slow intravenous infusion, or intramuscular injection. ondansetron hydrochloride. Ondansetron Dilution Globalrph.
From www.realvalueproducts.com
ONDANSETRON HYDROCHLORIDE ORAL SOLUTION SOL 4MG/5ML 50ML Real Value Rx Ondansetron Dilution Globalrph the first dose is infused over 15 minutes beginning 30 minutes before the start of emetogenic chemotherapy. dilutions of ondansetron in sodium chloride 0.9%w/v or in glucose 5%w/v have been demonstrated to be. dilution of ondansetron injection in 50 ml of 5% dextrose injection or 0.9% sodium chloride injection is required before. this reference contains standard. Ondansetron Dilution Globalrph.
From fda.report
ONDANSETRON HYDROCHLORIDE ondansetron solution Ondansetron Dilution Globalrph dilution of ondansetron injection in 50 ml of 5% dextrose injection or 0.9% sodium chloride injection is required before. Prevention of nausea and vomiting. ondansetron can be administered as a slow intravenous injection or slow intravenous infusion, or intramuscular injection. the first dose is infused over 15 minutes beginning 30 minutes before the start of emetogenic chemotherapy.. Ondansetron Dilution Globalrph.
From fda.report
ONDANSETRON ondansetron hydrochloride injection, solution Ondansetron Dilution Globalrph Prevention of nausea and vomiting. this reference contains standard dilutions including iv admixture drug concentration, infusion volumes, and. dosage adjustment for patients with impaired hepatic function: the first dose is infused over 15 minutes beginning 30 minutes before the start of emetogenic chemotherapy. ondansetron can be administered as a slow intravenous injection or slow intravenous infusion,. Ondansetron Dilution Globalrph.
From pharmbaba.com
Ondansetron Oral Solution IP Uses, Side effects PharmBaba Ondansetron Dilution Globalrph dosage adjustment for patients with impaired hepatic function: this reference contains standard dilutions including iv admixture drug concentration, infusion volumes, and. Prevention of nausea and vomiting. ondansetron hydrochloride should be diluted in 5% dextrose or 0.9% sodium chloride or other compatible infusion fluid (see. ondansetron can be administered as a slow intravenous injection or slow intravenous. Ondansetron Dilution Globalrph.
From fda.report
ONDANSETRON ondansetron hydrochloride injection, solution Ondansetron Dilution Globalrph ondansetron hydrochloride should be diluted in 5% dextrose or 0.9% sodium chloride or other compatible infusion fluid (see. dosage adjustment for patients with impaired hepatic function: this reference contains standard dilutions including iv admixture drug concentration, infusion volumes, and. the injection formulation should be diluted prior to iv administration. Prevention of nausea and vomiting. dilutions. Ondansetron Dilution Globalrph.
From www.indiamart.com
Ondansetron Hydrochloride Oral Solution IP 2 mg at Rs 37/bottle Ondansetron Dilution Globalrph dilution of ondansetron injection in 50 ml of 5% dextrose injection or 0.9% sodium chloride injection is required before. ondansetron hydrochloride should be diluted in 5% dextrose or 0.9% sodium chloride or other compatible infusion fluid (see. dilutions of ondansetron in sodium chloride 0.9%w/v or in glucose 5%w/v have been demonstrated to be. ondansetron can be. Ondansetron Dilution Globalrph.
From tajpharma.in
Ondansetron 2mg, 5ml Oral Solution FDA approved Taj Pharma India Ondansetron Dilution Globalrph the first dose is infused over 15 minutes beginning 30 minutes before the start of emetogenic chemotherapy. this reference contains standard dilutions including iv admixture drug concentration, infusion volumes, and. dilution of ondansetron injection in 50 ml of 5% dextrose injection or 0.9% sodium chloride injection is required before. the injection formulation should be diluted prior. Ondansetron Dilution Globalrph.
From cofarca.net
Ondansetron 2 mg/1ml Solución Inyectable COFARCA Ondansetron Dilution Globalrph ondansetron hydrochloride should be diluted in 5% dextrose or 0.9% sodium chloride or other compatible infusion fluid (see. this reference contains standard dilutions including iv admixture drug concentration, infusion volumes, and. the injection formulation should be diluted prior to iv administration. dilution of ondansetron injection in 50 ml of 5% dextrose injection or 0.9% sodium chloride. Ondansetron Dilution Globalrph.
From dailymed.nlm.nih.gov
DailyMed ONDANSETRON HYDROCHLORIDE ondansetron hydrochloride tablet Ondansetron Dilution Globalrph this reference contains standard dilutions including iv admixture drug concentration, infusion volumes, and. dilution of ondansetron injection in 50 ml of 5% dextrose injection or 0.9% sodium chloride injection is required before. dosage adjustment for patients with impaired hepatic function: ondansetron can be administered as a slow intravenous injection or slow intravenous infusion, or intramuscular injection.. Ondansetron Dilution Globalrph.
From www.drugs.com
Ondansetron Injection FDA prescribing information, side effects and uses Ondansetron Dilution Globalrph Prevention of nausea and vomiting. the first dose is infused over 15 minutes beginning 30 minutes before the start of emetogenic chemotherapy. dilutions of ondansetron in sodium chloride 0.9%w/v or in glucose 5%w/v have been demonstrated to be. dosage adjustment for patients with impaired hepatic function: this reference contains standard dilutions including iv admixture drug concentration,. Ondansetron Dilution Globalrph.
From www.indiamart.com
Ondansetron Oral Solution IP at Rs 65/bottle Azamshah Layout Nagpur Ondansetron Dilution Globalrph ondansetron hydrochloride should be diluted in 5% dextrose or 0.9% sodium chloride or other compatible infusion fluid (see. dosage adjustment for patients with impaired hepatic function: the injection formulation should be diluted prior to iv administration. this reference contains standard dilutions including iv admixture drug concentration, infusion volumes, and. dilution of ondansetron injection in 50. Ondansetron Dilution Globalrph.
From www.indiamart.com
Ondansetron Hydrochloride Oral Solution IP, for Personal, Packaging Ondansetron Dilution Globalrph ondansetron hydrochloride should be diluted in 5% dextrose or 0.9% sodium chloride or other compatible infusion fluid (see. ondansetron can be administered as a slow intravenous injection or slow intravenous infusion, or intramuscular injection. Prevention of nausea and vomiting. the first dose is infused over 15 minutes beginning 30 minutes before the start of emetogenic chemotherapy. . Ondansetron Dilution Globalrph.
From www.mcguffmedical.com
Ondansetron 2mg/mL MDV 20mL/Vial McGuff Medical Products Ondansetron Dilution Globalrph ondansetron hydrochloride should be diluted in 5% dextrose or 0.9% sodium chloride or other compatible infusion fluid (see. the injection formulation should be diluted prior to iv administration. Prevention of nausea and vomiting. the first dose is infused over 15 minutes beginning 30 minutes before the start of emetogenic chemotherapy. dilutions of ondansetron in sodium chloride. Ondansetron Dilution Globalrph.
From www.drugs.com
Ondansetron Oral Solution FDA prescribing information, side effects Ondansetron Dilution Globalrph the injection formulation should be diluted prior to iv administration. ondansetron hydrochloride should be diluted in 5% dextrose or 0.9% sodium chloride or other compatible infusion fluid (see. dosage adjustment for patients with impaired hepatic function: Prevention of nausea and vomiting. the first dose is infused over 15 minutes beginning 30 minutes before the start of. Ondansetron Dilution Globalrph.
From ndclist.com
NDC 687888365 Ondansetron Solution Oral Label Information Details Ondansetron Dilution Globalrph the injection formulation should be diluted prior to iv administration. dosage adjustment for patients with impaired hepatic function: this reference contains standard dilutions including iv admixture drug concentration, infusion volumes, and. ondansetron can be administered as a slow intravenous injection or slow intravenous infusion, or intramuscular injection. ondansetron hydrochloride should be diluted in 5% dextrose. Ondansetron Dilution Globalrph.